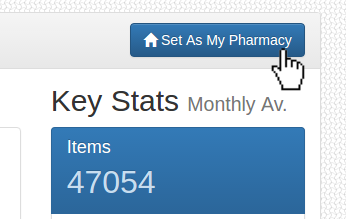
Pharmacy Not Set
Please use the search function to find your pharmacy, then click 'Set As My Pharmacy'. This can also be modified in the Settings page.

This site is intended for Healthcare Professionals only
2021-06-16 10:00:08 (5 year(s) ago)
Zentiva Pharma UK Limited is recalling the above batch of Co-codamol 30/500 Effervescent Tablets as a precautionary measure due to an issue with the homogeneity of the batch. This issue means that there is the potential…