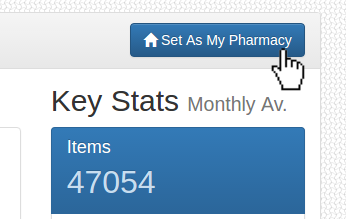
Pharmacy Not Set
Please use the search function to find your pharmacy, then click 'Set As My Pharmacy'. This can also be modified in the Settings page.

This site is intended for Healthcare Professionals only
2019-11-28 14:14:58 (6 year(s) ago)
Pharmaswiss ÄŒeska republika s.r.o. (an affiliate of Bausch & Lomb UK Limited) is recalling all unexpired batches of Emerede Adrenaline Autoinjectors after identifying an error than can cause some pens to fail to activate.